
September 21st marks World Alzheimer’s Day, a global opportunity to raise awareness about Alzheimer’s disease, the most common cause of dementia worldwide. On this day, we honor those affected and shed light on how genetics can play a role in the development of the disease. Today, we’ll explore the case of Pooja, a 68-year-old woman navigating the challenges of Alzheimer’s, with a focus on the genetic factors that might influence the condition.
Pooja is a 68-year-old retired schoolteacher. Over the past two years, her family has noticed worrying changes in her behavior. Once an organized and vibrant individual, she now struggles with simple tasks like managing her finances and remembering appointments. More recently, she has been getting lost in familiar places and repeating the same questions in conversations. Her children have been concerned by these symptoms and decided it was time to get help. When they sat down with her doctor, it became clear that she was showing early signs of Alzheimer’s Disease (AD). What worried the family even more was their knowledge of Pooja’s father’s battle with Alzheimer’s, which began in his early 70s. Two of her paternal aunts also showed signs of cognitive decline, raising red flags about a possible genetic connection.
What is Alzheimer’s Disease? Let us understand the basics.
Alzheimer’s disease is a neurodegenerative condition that leads to progressive memory loss, confusion, and changes in behavior. It’s the leading cause of dementia and affects millions of people worldwide. While there is no cure for Alzheimer’s, early diagnosis can help manage symptoms and plan for future care.
There are two primary forms of Alzheimer’s disease:
- Early-onset Alzheimer’s Disease (EOAD): Occurs before age 65 and is often linked to specific genetic mutations.
- Late-onset Alzheimer’s Disease (LOAD): Typically occurs after age 65 and is influenced by both genetics and environmental factors.
Her case aligns with Late-Onset Alzheimer’s Disease (LOAD), given her age and the gradual development of her symptoms.
Could Genetics Be at Play?
With her family history of Alzheimer’s, Pooja’s children wanted to know more about the role genetics could play in their mother’s condition—and their own potential risk.
One of the most well-studied genes related to Alzheimer’s is APOE, specifically the APOE ε4 allele. Carrying one or two copies of the APOE ε4 variant significantly increases the risk of developing Alzheimer’s disease, however, it’s important to note that not everyone with this allele develops the condition, and many who don’t carry it may still be affected.
In Pooja’s case, her family history suggests a possible genetic predisposition to Alzheimer’s, particularly linked to the APOE gene. However, other genetic factors—along with lifestyle and environmental influences—can also play a role.
What Genetic Testing Can Tell Us?
Pooja’s children, understandably worried about their own future risk of developing Alzheimer’s, are exploring the option of genetic testing. Genetic testing for Alzheimer’s Disease can provide some insight into a person’s risk, but it’s important to remember that genetics isn’t destiny. For Pooja, testing could confirm if she carries the APOE ε4 variant, helping her family understand more about the genetic component of her condition.
Testing might also be useful for her children, but there are critical considerations before pursuing predictive genetic testing:
- Ethical concerns: Would knowing their genetic risk affect their life choices or create anxiety?
- Psychological impact: How would they cope with the knowledge that they may be at higher risk of developing Alzheimer’s?
- No certainty: Even if they carry the APOE ε4 allele, it doesn’t mean they will develop Alzheimer’s. Similarly, some without the allele still get the disease.
While the test can give useful insights, it can’t provide definitive answers. THIS IS WHY WE EMPHASIZE THE IMPORTANCE OF A PRE-TEST GENETIC COUNSELLING SESSION.
How to Reduce Alzheimer’s Risk: It's Not Just About Genes
While genetics plays a role in Alzheimer’s disease, especially in families like Pooja’s, there are lifestyle factors that can influence the risk:
- Exercise: Regular physical activity, especially aerobic exercise, has been shown to benefit brain health.
- Diet: A heart-healthy diet, such as the Mediterranean diet, can also help protect the brain.
- Mental engagement: Keeping the brain active through learning new skills, reading, and socializing can promote cognitive health.
- Managing cardiovascular health: Conditions like hypertension, diabetes, and high cholesterol can increase Alzheimer’s risk, so managing these is key.
For Pooja’s children and others worried about Alzheimer’s, incorporating these healthy habits could help reduce their risk—whether or not they carry the APOE ε4 allele.
Finally, to conclude, for many families, genetics may offer clues, but it’s only part of the picture. Raising awareness about Alzheimer’s, the importance of early diagnosis, and the role of genetic and lifestyle factors can help families make informed decisions about their health.
If you’re concerned about Alzheimer’s in your family, consider speaking to a genetic counselor. They can guide you through testing options, help you understand your risk, and suggest ways to reduce it.
Remember: While Alzheimer’s can’t yet be cured, understanding its genetic and lifestyle components empowers us to take proactive steps for brain health.
References :
MyDNA

Imagine a young boy named Ayaan growing up in a friendly neighborhood in Mumbai. He's full of energy, and always eager to play cricket with his friends whenever he gets a chance. But right before his fifth birthday, his parents begin to notice something different about him. They notice that he struggles to keep up while playing games, falls more often, and seems unusually tired. They get worried and take him to a doctor, where they're introduced to the world of Duchenne Muscular Dystrophy (DMD).
What is Duchenne Muscular Dystrophy?
Duchenne Muscular Dystrophy is a genetic disorder that leads to progressive muscle weakness and degeneration predominantly affecting male children. This disease is rapidly progressive and the incidence is approximately 1 in 3500 live male births in the world. It's caused by a mutation in the dystrophin gene, which is crucial for maintaining the structural integrity of muscle cells. Without a functioning dystrophin, the muscles weaken over time.
What are the Symptoms?
In Ayaan’s case, the symptoms started with difficulty in activities that other children his age found easy. His parents noticed he had trouble climbing stairs and struggled with running. His once-energetic demeanor began to change, as muscle weakness started to affect his ability to walk and play. By the age of seven, Ayaan was using a wheelchair for longer distances, and his family noticed that his calves had become unusually large—an effect of the disease known as pseudohypertrophy.
As the disease progresses, it can also affect the muscles responsible for breathing and heart function. For Ayaan, this means regular visits to specialists and ongoing monitoring to manage potential respiratory and cardiac issues.
How can you diagnose DMD?
For Ayaan, diagnosing DMD involved a series of steps:
- Clinical Examination: The pediatrician noted the characteristic signs of muscle weakness and an enlarged calf muscle.
- Genetic Testing: Genetic Testing using the technique of MLPA can identify the presence of a mutation in the dystrophin gene.
- Muscle Biopsy: This step usually can provide additional confirmation by examining muscle tissue.
- Blood Tests: Elevated creatine kinase levels were observed, indicating muscle damage.
Let's understand the Treatment and Management...
While there’s no cure for DMD yet, there are several ways to manage the symptoms and enhance quality of life:
- Medications: In Ayaan’s case, his doctors recommended corticosteroids to slow the progression of muscle degeneration and improve his strength and function.
- Physical Therapy: Regular physical therapy sessions are essential to maintain mobility and reduce the risk of contractures. Ayaan’s physical therapist helps him with exercises tailored to his needs.
- Assistive Devices: Ayaan uses a wheelchair for longer distances, and braces help support his legs and keep him comfortable.
- Respiratory Support: As the disease progresses, Ayaan may need ventilatory support to assist with breathing, although his parents hope for advancements in treatment that could delay this necessity.
Advances in Research
Gene therapy is proving promising for treating Duchenne Muscular Dystrophy (DMD). This innovative approach aims to correct or replace the faulty dystrophin gene responsible for the disease, potentially transforming the future of treatment. In India, renowned institutions and research facilities are at the forefront of this groundbreaking work, working to advance gene therapy techniques and bring new hope to patients and their families.
Living with DMD in India
For families like Ayaan, living with DMD involves navigating a complex healthcare landscape. Access to specialized care and resources can vary widely. In urban cities like Mumbai, families may have better access to medical professionals and support networks, but in rural regions, the challenges can be more pronounced.
Support organizations and advocacy groups play a crucial role in providing information, emotional support, and resources. In India, organizations such as the Muscular Dystrophy Foundation India (MDFI) work tirelessly to support individuals with muscular dystrophy and their families. There is also a Molecular Diagnostics Counseling Care and Research Centre (MDCRC) is a not-for-profit NGO working on Neuromuscular genetic disorders viz. Duchenne Muscular Dystrophy (DMD). They offer valuable resources, connect families with experts, and advocate for increased research and awareness.
Genetic Counselling for DMD
Genetic counseling provides invaluable support for families affected by Duchenne Muscular Dystrophy. It helps parents gain a clear understanding of several critical aspects:
- Understanding the Disorder: Genetic counseling explains the causes and various types of muscular dystrophy, providing clarity on how these conditions arise.
- Recognizing Symptoms and Progression: Counselors provide information on the typical symptoms and progression of DMD/BMD, helping families anticipate the course of the disorder.
- Assessing Recurrence Risk: Families are informed about the likelihood of the disorder recurring in future children, which aids in understanding their genetic risk.
- Family Planning: Genetic counseling offers guidance on family planning options, helping parents make informed decisions about future pregnancies.
- Carrier Testing: The importance of carrier testing is emphasized, which is crucial for identifying whether family members carry the genetic mutations associated with these conditions.
- Interpreting Genetic Test Results: Counselors assist in understanding genetic test results and provide advice on any additional testing that may be needed.
Conclusion
Duchenne Muscular Dystrophy is a challenging condition, but stories like Ayaan’s remind us of the strength and resilience that families exhibit every day. With ongoing research and support, there’s hope for better treatments and, one day, a cure. By staying informed and connected with support networks, we can help improve the lives of those affected by DMD and work towards a future where this condition no longer holds back the dreams and aspirations of individuals like Aarav.
References :
Nil

Background
A 45-year-old female with a history of anxiety and depression has been struggling to find effective medication management. Over several years, she has tried multiple antidepressants and anxiolytics but experienced a mix of poor efficacy and significant side effects, such as fatigue, dizziness, and increased anxiety. After numerous unsuccessful attempts at finding the right medication, her doctor recommended pharmacogenomic testing to tailor her treatment.
Key Issues
- Medication Efficacy and Safety: The patient experienced limited improvement with multiple medications while enduring adverse effects.
- Trial-and-Error Prescribing: Repeated medication switches without clear guidance led to frustration and a prolonged untreated state.
- Patient Quality of Life: The ongoing medication trials and adverse effects led to worsening mental health and reduced daily functioning.
Pharmacogenomic Testing and Its Role
The patient underwent pharmacogenomic testing, which analyzed several genes known to influence drug metabolism and response, including:
- CYP2D6 and CYP2C19: Genes encoding enzymes involved in metabolizing many antidepressants.
- SLCO1B1: Associated with drug transport and can impact statin-related muscle toxicity.
- SLC6A4 (serotonin transporter gene): Variants of this gene are linked to response variability in selective serotonin reuptake inhibitors (SSRIs).
Test Findings
The pharmacogenomic test revealed:
- CYP2D6 Poor Metabolizer: The patient had a genetic variant resulting in reduced CYP2D6 enzyme activity, leading to slower metabolism of certain antidepressants, such as tricyclic antidepressants (TCAs) and SSRIs like fluoxetine. This explained the side effects she experienced even at lower doses.
- CYP2C19 Ultra-Rapid Metabolizer: Increased enzyme activity for this gene meant she metabolized certain SSRIs, like citalopram, too quickly, leading to subtherapeutic levels and reduced efficacy.
- SLC6A4 L/L Genotype: This genotype is associated with a better response to SSRIs, but given the patient’s metabolism profiles, conventional SSRIs may not be optimal.
Clinical Outcome
Based on these results, the patient’s provider switched her medication to venlafaxine, an antidepressant metabolized primarily through pathways that were not impacted by her CYP2D6 or CYP2C19 status. The dosing was also adjusted to account for her pharmacogenomic profile.
Within weeks, the patient reported a marked improvement in her anxiety and depression symptoms with minimal side effects. Over the following months, she continued to improve with no need for further medication changes. Her quality of life and daily functioning returned to near-normal levels.
Summary and Recommendations
Pharmacogenomic testing provided crucial insights into this patient’s unique drug metabolism profile, allowing for personalized adjustments to his medication regimen. The intervention led to better treatment outcomes and fewer side effects, demonstrating the value of integrating pharmacogenomics into clinical practice.
Next Steps:
- Continued monitoring and possible updates to the medication plan as new pharmacogenomic data become available.
- Consideration of pharmacogenomic testing for close family members who might share similar metabolic profiles.
This case highlights how targeted pharmacogenomic testing can lead to significant improvements in personalized care, particularly in patients with complex medication needs.
References :
mydna.lordsmicrobiotech.com

- Disrupting gene regulation: Some SNPs might fall within regulatory regions of genes, affecting how much protein is produced or how the gene functions. This can lead to altered cellular processes that contribute to cancer development.
- Affecting protein function: In some cases, the SNP might directly change the amino acid sequence of a protein, potentially affecting its function and impacting testicular development or germ cell function.
References :
Breakdown of the affected communities:
- Central India: This region has the highest concentration, with communities like Pardhi, Bharia, Bhil, Gond, an
d Korku having a significant number of carriers and cases. - Western India: Tribal communities in Gujarat, Maharashtra, and Rajasthan also show a high prevalence. Examples include Warli, Koli, Dangi, and Halba communities.
- Southern India: Some southern states like Andhra Pradesh, Telangana, Karnataka,
and Tamil Nadu have tribal communities with a high sickle cell burden. These include Chenchu, Gond, and Particularly Vulnerable Tribal Groups (PVTGs).
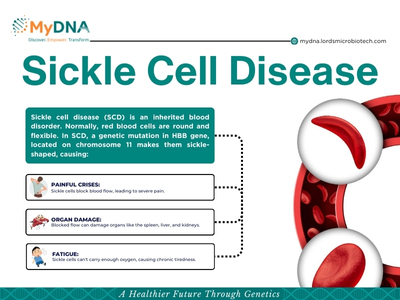
- Mutation: The most common mutation in sickle cell disease is a single change in the DNA code of the HBB gene. This single change alters one amino acid (the building block of proteins) in the beta-globin protein.
- Hemoglobin S: This specific mutation creates an abnormal form of hemoglobin called hemoglobin S (HbS).
- Inheritance: Sickle cell disease is an autosomal recessive disorder. This means that a person needs to inherit two copies of the mutated gene, one from each parent, to develop the disease.
- Parents with one copy of the mutated gene (carriers) typically don't have sickle cell disease themselves.
- Children who inherit one normal HBB gene and one HbS gene have a condition called sickle cell trait. They are carriers but don't have the disease.
References :

- KLK3 Gene: This gene specifically codes for the production of PSA. Variations in this gene can lead to naturally higher or lower PSA levels even in healthy men.
- Multiple Loci: Researchers have identified over 40 different locations (loci) in the genome that influence PSA levels.These variations are often subtle, but combined, they can significantly impact a man's baseline PSA.
- Heredity: A family history of prostate cancer can also be linked to PSA levels. Genes like BRCA1, BRCA2, and HOXB13, associated with hereditary prostate cancer risk, might also influence PSA production.
Here are some key things to know about PSA levels:
- A PSA level of 4 ng/mL (nanograms per milliliter) or lower is generally considered normal.
- A PSA level between 4 and 10 ng/mL is considered borderline and may warrant further testing.
- A PSA level of 10 ng/mL or higher is considered high and may indicate prostate cancer, although there are other possible explanations.
References :
Understanding the Genetics of ADHD
Attention deficit hyperactivity disorder (ADHD) is a common neurodevelopmental condition that affects millions of children and adults worldwide.The symptoms of impulsivity, hyperactivity, and inattention are what define ADHD. The prevalence of children ever diagnosed with ADHD increased by 42% between 2003 (7.8%) and 2011 (11.0%). Males had a consistently higher prevalence of ADHD than females.
Some people may primarily suffer from inattention, which makes it difficult for them to focus, organize their work, or obey directions. Some people may be hyperactive and impulsive, which can lead to restlessness, impulsive decision-making.
Family Linkage
Research strongly suggests a hereditary component to ADHD. Studies on twins show a high concordance rate, meaning if one twin has ADHD, the other has a significantly increased risk of developing it as well. Family studies also reveal a pattern – children with a parent or sibling diagnosed with ADHD are much more likely to have it themselves.
Complexity of ADHD
Unlike some conditions caused by a single gene mutation, ADHD is believed to be polygenic. This means multiple genes, each with a small influence, interact to increase susceptibility.
Here are some of the genes that have been linked to ADHD:
- Dopamine transporter (DAT1) gene: This gene regulates the dopamine system, which is involved in attention, focus, and reward processing.
- Dopamine receptor D4 (DRD4) gene: This gene is another important player in the dopamine system. Certain variations of this gene have been associated with an increased risk of ADHD.
- Serotonin transporter (SERT) gene: Serotonin is a neurotransmitter involved in mood regulation and impulse control. Variations in the SERT gene have also been linked to ADHD.
- FOXP2 gene: This gene is important for speech and language development. Mutations in FOXP2 have been linked to both ADHD and language disorders.
While genetics play a significant role, Environmental factors can also influence the development and expression of ADHD. Prenatal exposure to toxins, childhood trauma, and even diet can have significant impact on the individual with the condition.
The genetic link to ADHD doesn't mean it's inevitable. If you have a family history of the condition, it's wise to be aware of the signs and symptoms. Early diagnosis and intervention can make a big difference in managing ADHD effectively.
A few misconceptions about ADHD:
- ADHD is only a lack of discipline: Despite what the general public believes, ADHD is more complex than just a lack of willpower or discipline. It is a neurological disorder influenced by both hereditary and environmental factors.
- ADHD is limited to children: Although it is frequently identified in childhood, ADHD can continue throughout adolescence and maturity. Even though many adults did not receive a diagnosis when they were younger, they now deal with symptoms that impact different areas of their lives.
- Medication is the only available treatment: Medication is not the only choice for treating ADHD symptoms, even though it can be a useful aid in managing them. In addition to behavioral therapy, organizational techniques, lifestyle modifications, and support systems are helpful in the management of ADHD.
- People with ADHD lack intelligence: Intelligence is unaffected by ADHD. In actuality, a lot of people with ADHD have special talents including creativity, inventiveness, and problem-solving skills.
Assisting with ADHD:
- Education and Awareness: Getting educated on ADHD can help debunk myths and lessen stigma for both oneself and others. Communities that are aware of the difficulties and assets related to ADHD are more empathetic and supportive of one another.
- Individualized Strategies: Since everyone with ADHD is affected differently, it's critical to create individualized symptom management plans. This could entail using organizational tools, creating routines, breaking things down into smaller pieces, and setting reasonable goals.
- Medication Management: For those who choose to pursue medication, working closely with healthcare professionals to find the right medication and dosage is essential. Regular monitoring and adjustments may be necessary to optimize treatment outcomes.
- Behavioral Therapy: Cognitive-behavioral therapy (CBT) and other forms of psychotherapy can help individuals with ADHD develop coping mechanisms, improve time management skills, and address co-occurring conditions such as anxiety or depression.
- Support Networks: Making friends with people who also have ADHD or attending support groups can offer empathy, encouragement, and useful advice on how to deal with day-to-day difficulties. Local support groups, online networks, and therapeutic groups can provide helpful links and motivation.
In summary
Due to its complexity and variety, ADHD has to be managed and supported in a comprehensive manner. People with ADHD can flourish and realize their full potential by accepting empowerment techniques, dispelling myths, and comprehending the facts of the condition.
References :

Schizophrenia
Schizophrenia is a brain disorder classified as a psychosis which is characterised with symptoms like false perceptions called hallucinations, decreased ability to function, Inappropriate emotional reactions, erratic speech and behavior, trouble with daily activities and personal cleanliness, disordered thinking and concentration. Genetics of schizophrenia is multifaceted, affecting 24 million individual worldwide with each genetic variation and epigenetic alteration contributing to the disorder.
Genetics (vs environmental) factors plays a predominant role in the development of schizophrenia, with heritability estimates ranging from 60% to 80%. However this condition does not follow specific dominant or recessive pattern of inheritance.
Candidate Genes
Several candidate genes have emerged as key players in schizophrenia susceptibility. Among these, the dopamine-related genes, such as DRD2 and COMT, has been given considerable attention due to their involvement in neurotransmission and dopaminergic dysfunction, a hallmark feature of schizophrenia.
Additionally, genes involved in synaptic plasticity (e.g., DISC1, NRXN1) and glutamatergic neurotransmission (e.g., GRIN2A, GRM3) have been implicated, underscoring the complexity of neuronal signaling disruptions in schizophrenia pathogenesis.
Polygenic Risk Ratings: Linking the Pieces
Recent developments in polygenic risk score (PRS) analysis have made it possible to gain a more thorough understanding of the genetics of schizophrenia. Through the combination of genetic variants across the genome, PRS offers insights into the susceptibility of each individual to schizophrenia. Additionally, PRS makes it easier to identify genetic risk that is common to psychiatric diseases, revealing biological pathways that overlap and possible targets for treatment.
Pathways and Networks: Researchers are pinpointing specific gene networks involved in brain development and function that seem to be disrupted in schizophrenia. These networks are often related to:
- Glutamate and GABA signaling: Neurotransmitters crucial for communication between brain cells.
- Synaptic function: How brain cells connect and transmit information.
- Neurodevelopment: Processes shaping the brain's structure.
Environmental Triggers: Genes alone don't dictate who develops schizophrenia. Environmental factors like childhood trauma, substance abuse, and social isolation can interact with genetic predispositions, potentially triggering the onset of the illness.
Future Direction :
Risk Prediction: It helps to understanding an individual's genetic makeup could potentially provide an estimate of their susceptibility to schizophrenia.
As we unravel the genetic intricacies of schizophrenia, personalized approaches to diagnosis and treatment are on the horizon. Integrating genetic data with clinical parameters and neuroimaging techniques holds promise for delineating distinct subtypes of schizophrenia and guiding tailored therapeutic interventions.\
In summary, we are now a inch closer to understanding individuals genetic code for schizophrenia. While much remains to be elucidated, ongoing research endeavors by technological advancements offer unprecedented opportunities to the molecular understanding of schizophrenia and has shaped the way for innovative treatment strategies, where interventions are tailored to the individual's genetic makeup and underlying neurobiology, ultimately offering hope for improved outcomes and quality of life for individuals with this complex condition.
References :

Bipolar disorder, characterised by its distinct mood swings between mania and depression, affects 46 million globally. Bipolar disorder often emerges during adolescence and early adulthood. However, symptoms can appear much earlier, sometimes in childhood. It has long been recognized as a complex interplay between genetic predisposition and environmental factors.
Lack of Self-Awareness:
During manic episodes, individuals may lack insight into their own behaviour. They might feel invincible, make impulsive decisions, or engage in risky activities without fully understanding the consequences.
Stigma and Misconceptions:
There's a lot of misunderstanding surrounding bipolar disorder. People often confuse it with moodiness or simply being "dramatic." This can make it difficult for individuals with the condition to seek help or feel validated.
Family History : The Intertwined Threads
Family history paints a clear picture: individuals with a close relative diagnosed with bipolar disorder have a significantly higher risk of developing the condition themselves. This suggests a strong genetic influence. However, understanding it is not as simple as a single gene mutation. Bipolar disorder is considered a polygenic condition, meaning multiple genes, each with small effects, contribute to the overall risk.
Genes on the Radar:
Genetic advancement have helped identify several genes associated with bipolar disorder, including:
- ANK3: Mutations in this gene have been linked to bipolar disorder, with possible implications for brain development and function.
- PALB2 : The specific SNP associated with bipolar disorder might alter the function of the PALB2 protein, potentially impacting these critical cellular processes. This altered function could be related to the development of bipolar disorder.
- LAMP3 : LAMP3, or lysosomal-associated membrane protein 3, plays a role in cellular processes. Research suggests it to be involved in cell death (apoptosis).
- DGKH : DGKH has also been identified as the strongest Panic Disorder associated gene. It has also been associated with altered brain activity in regions involved in mood regulation, potentially contributing to the development of bipolar disorder.
Beyond the Genes: The Environmental Trigger
While genetics provide a foundation for susceptibility, environmental factors act as triggers that can push an individual towards developing bipolar disorder. These can include:
- Stressful life events: Traumatic experiences or significant life changes can exacerbate mood imbalances.
- Substance abuse: Drug and alcohol use can worsen symptoms and potentially trigger episodes.
- Early life experiences: Childhood trauma or neglect may increase vulnerability to the disorder.
The Evolving Landscape of Genetic Research:
Understanding the genetic basis of bipolar disorder holds immense potential for the future:
- Improved Diagnosis: Genetic markers could aid in earlier identification of individuals at high risk, allowing for preventative measures or early intervention strategies.
- Personalized Treatment: Tailored treatment approaches based on an individual's genetic profile could lead to more effective management of the condition.
While the path to a definitive cure remains long, unraveling the genetics of bipolar disorder is crucial for advancements in diagnosis, treatment, and ultimately, improving the lives of those affected by this complex mental health condition.
References :

PKU is an inherited metabolic disorder affects approximately 1 in every 10,000 to 15,000 newborns worldwide. It is characterised by the body's inability to properly metabolise the amino acid phenylalanine. Normally, an enzyme called phenylalanine hydroxylase (PAH) converts phenylalanine into another amino acid, tyrosine. The high levels of Phenylalanine present in PKU competitively inhibits the hydroxylation of tyrosine by tyrosinase which is the first step in the formation of the pigment melanin. However, individuals with PKU lack this enzyme or have a defective version, leading to a buildup of phenylalanine in the blood and brain. Virtually all untreated patients have IQ below 50. Despite its rarity, PKU can have significant and lifelong consequences if left untreated.
Causes of PKU:
PKU is caused by mutations in the gene responsible for producing the PAH enzyme. This gene is inherited in an autosomal recessive pattern, meaning that a child must inherit two mutated copies of the gene (one from each parent) to develop the disorder. If both parents are carriers of the mutated gene, there's a 25% chance with each pregnancy that their child will have PKU.
Symptoms of PKU:
Symptoms of PKU typically appear within the first few months of life, after the baby begins to consume phenylalanine-containing foods, such as breast milk or formula. These symptoms may include:
- Developmental delays.
- Intellectual disability.
- Behavioural problems.
- Seizures.
- Skin rashes (eczema).
- Lighter skin, hair, and eyes compared to family members.
- Musty odour in the breath, skin, and urine due to the buildup of phenylalanine byproducts.
Maternal PKU :
When women with PKU who are not on a low phenylalanine diet become pregnant, the offspring are affected with maternal PKU syndrome. Congenital cardiac abnormalities, mental retardation, and microcephaly are caused by high blood levels in the mother. Thus, phenylalanine needs to be controlled with diet starting before conception.
Diagnosis of PKU:
PKU is usually detected through newborn screening tests, which are performed shortly after birth. These tests involve collecting a small blood sample from the baby's heel and analysing it for elevated levels of phenylalanine. If PKU is suspected based on the screening results, further diagnostic tests, such as genetic testing or blood phenylalanine levels, may be conducted to confirm the diagnosis.
Treatment and Management:
Early diagnosis and treatment are crucial in managing PKU and preventing complications. The primary goal of treatment is to maintain blood phenylalanine levels within a safe range to prevent brain damage and cognitive impairment. Treatment options for PKU include:
- Dietary restrictions: The cornerstone of PKU management involves following a strict low-phenylalanine diet. This typically means avoiding high-protein foods, such as meat, dairy, eggs, nuts, and certain grains, and consuming specially formulated medical foods and formulas that are low in phenylalanine.
- Supplementary therapy: In addition to dietary restrictions, individuals with PKU may require supplementation with specific amino acids, vitamins, and minerals to ensure proper nutrition.
- Monitoring: Regular monitoring of blood phenylalanine levels is essential to adjust dietary therapy and ensure that phenylalanine levels remain within the target range.
- Lifelong management: PKU is a lifelong condition, and individuals with PKU require ongoing medical supervision and support to manage their condition effectively.
Conclusion:
Phenylketonuria (PKU) is a genetic disorder that affects the body's ability to metabolise the amino acid phenylalanine. Without proper treatment, PKU can lead to serious neurological and developmental problems. However, with early diagnosis and lifelong management, individuals with PKU can lead healthy and fulfilling lives. Increased awareness, improved screening methods, and ongoing research into new treatment options are essential in improving outcomes for individuals living with PKU.
References :

In the intricate tapestry of human genetics, certain conditions stand out for their widespread impact and intriguing history. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is one such condition, affecting millions worldwide and holding significant implications for health, particularly in regions with a high prevalence of malaria.
The Basics of G6PD Deficiency
G6PD is an enzyme encoded by the G6PD gene, located on the X chromosome. It plays a crucial role in red blood cell function by protecting them from oxidative damage. However, individuals with G6PD deficiency have lower levels or defective forms of this enzyme, making their red blood cells more vulnerable to oxidative stress.
Global Distribution and Epidemiology
G6PD deficiency is one of the most common enzyme deficiencies globally, affecting approximately 400 million people. Its prevalence is particularly high in regions such as Africa, the Mediterranean, the Middle East, and parts of Asia. Interestingly, the distribution of G6PD deficiency overlaps with regions where malaria is endemic.
The Malaria Connection
The relationship between G6PD deficiency and malaria has long intrigued researchers. Studies have shown that individuals with G6PD deficiency may have some degree of protection against malaria. This is hypothesized to occur because the lower levels of red blood cells in individuals with G6PD deficiency create an environment less hospitable for the malaria parasite, Plasmodium.
Historical Insights
The story of G6PD deficiency dates back to the 1920s when Otto Warburg and his colleague first identified the enzyme's activity. However, it wasn't until the 1950s that Ernest Beutler and his team made significant breakthroughs, linking G6PD deficiency to episodes of acute hemolysis triggered by certain drugs, infections, and foods like fava beans.
Management and Treatment
While there is currently no cure for G6PD deficiency, management strategies focus on avoiding triggers of hemolytic crises and providing supportive care during acute episodes of anemia. In severe cases, blood transfusions may be necessary to alleviate symptoms and complications.
Raising Awareness and Promoting Understanding
As we delve deeper into the complexities of human genetics, it becomes increasingly important to raise awareness about conditions like G6PD deficiency. By understanding its global impact, historical context, and implications for health, we can better support affected individuals and work towards improved management and treatment strategies.
Conclusion
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is not just a genetic condition; it's a global concern with far-reaching implications. From its historical discovery to its intriguing connection with malaria, G6PD deficiency continues to captivate researchers and healthcare professionals alike. By shedding light on this condition, we can pave the way for greater understanding, support, and ultimately, improved outcomes for those affected.
References :
https://www.mydna.lordsmicrobiotech.com

Annually, Healthcare Decision Day stands as a call for individuals to assert control over their well-being and navigate healthcare decisions with clarity. Yet, amidst the vast array of options, many need clarification on misconceptions. Let's unravel these myths and embrace the role of genetic testing in shaping long-term health outcomes for families.
Myth vs. Fact: Unveiling Reality
Myth: Healthcare decisions react only to illness.
Fact: Healthcare involves proactive measures, preventive strategies, and personalized interventions for optimal well-being.
Myth: Genetic testing is exclusive to those with a familial disease history.
Fact: Genetic insights benefit everyone, guiding personalized healthcare decisions for individuals' empowerment.
Myth: Genetic testing is a one-time event with limited relevance.
Fact: Genetic testing is a lifelong investment, offering enduring insights into traits, disease risks, and evolving preventive measures.
Harnessing Genetic Potential: Strategic Investment
Genetic testing heralds a healthcare paradigm shift, providing personalized insights into genetic predispositions and empowering proactive health management. By decoding genetics, it offers a roadmap for prevention, early detection, and tailored interventions.
Through comprehensive genetic analysis, individuals identify health risks, optimize lifestyles, and implement preventive measures. Genetic testing transcends boundaries, offering actionable insights from cardiovascular health to cancer susceptibility.
Lifelong Commitment: Genetic Understanding
Contrary to belief, genetic understanding is lifelong, deepening in significance with scientific discoveries and evolving interventions. For families, it's foundational in comprehending inherited conditions, breaking disease cycles, and empowering future generations.
Embracing the Future: Unlocking Genetic Potential
On Healthcare Decision Day, let's transcend healthcare limitations and embrace genetic testing's potential. We have a healthier, resilient future by dispelling myths, embracing facts, and prioritizing preventive measures.
Let's make informed healthcare decisions, leveraging genetic testing to optimize well-being. Together, let's embark on a journey guided by genetic wisdom, fueled by the promise of a healthier tomorrow.
References :

Introduction
In recent years, India has found itself grappling with a mounting health crisis as cancer rates soar, earning it the dubious title of "the cancer capital of the world." A comprehensive report by the renowned Indian healthcare group, Apollo Hospitals, sheds light on the alarming prevalence of cancer and other non-communicable diseases across the nation, urging for urgent action.
The Alarming Statistics
The report paints a stark picture of the current health landscape, with skyrocketing cases of cancer and chronic conditions reaching critical levels. Despite reporting over a million new cases annually, India's cancer rate has not yet surpassed certain countries like Denmark, Ireland, and Belgium. However, it is currently lower than the U.S., recording 100 cases for every 100,000 people compared with 300 in the U.S.
The Growing Burden
Nevertheless, the situation is dire. The report highlights a concerning trend of rising pre-diabetes, prehypertension, and mental health disorders, signalling an impending healthcare crisis. Currently, one in three Indians is pre-diabetic, two in three are pre-hypertensive, and one in 10 struggles with depression. Chronic conditions like cancer, diabetes, hypertension, cardiovascular diseases, and mental health disorders have reached "critical levels."
Facts and Figures
- The number of cancer cases in India is expected to rise from 1.39 million in 2020 to 1.57 million by 2025.
- Among women, the most common forms of cancer are breast cancer, cervix cancer, and ovarian cancer. Among men, they are lung cancer, mouth cancer, and prostate cancer.
- India bucks the global trend with more women being diagnosed with cancer than men, according to a study published in the Lancet Oncology.
- Certain cancers are affecting younger people sooner than in other countries. For example, the median age for lung cancer in India is 59, compared to 70 in the U.S., 68 in China, and 75 in the U.K.
Genetic Insights: A Path to Prevention
One of the most promising avenues for managing the risk of cancer lies in genetic testing. By uncovering individuals' genetic predispositions to certain types of cancer, preventive strategies can be tailored to mitigate risks and promote early intervention.
MyDNA Onco Geneguard: Empowering Preventive Healthcare
In this context, MyDNA Onco Geneguard emerges as a powerful tool in the fight against cancer. This preventive genetic test analyses an individual's genetic profile to identify potential susceptibility to various types of cancer. By offering personalised insights and recommendations, it enables proactive measures to be taken, from lifestyle modifications to personalised screening protocols.
Embracing Innovation for a Healthier Tomorrow
As we confront the escalating challenge of cancer in India, it is imperative to embrace innovative solutions like genetic testing. By promoting awareness and encouraging early intervention, we can shift the narrative towards a future where cancer is not just treatable but preventable.
Take Charge of Your Health: Get Tested Today
Don't wait until it's too late. Take charge of your health journey by getting tested with MyDNA Onco Geneguard. Arm yourself with the knowledge you need to make informed decisions about your health and well-being. Together, let's pave the way towards a healthier, brighter future for generations to come.
In Conclusion
The fight against cancer in India is multifaceted, requiring a comprehensive approach that encompasses awareness, prevention, and early detection. Genetic testing, particularly with advancements like MyDNA Onco Geneguard, offers a promising avenue for proactive healthcare. By understanding our genetic predispositions and taking preventive measures, we can work towards a future where cancer is no longer a looming threat, but a preventable condition. Let's unite in this endeavour and pave the way towards a healthier India.
References :
https://time.com/6965528/india-cancer-capital-world-study/
https://www.cancer.gov/about-cancer/causes-prevention/genetics

As the sun climbs higher and the days longer, summer whispers the promise of outdoor escapades, leisurely moments by the shore, and delightful indulgences. Yet, amidst the allure of the season, safeguarding our wellness takes precedence, particularly in the face of summer-specific health hurdles.
Navigating Summer Wellness Through Genetics
Let's embark on a journey to uncover the transformative potential of understanding your genetic blueprint in steering you toward a summer brimming with vitality and well-being.
1. Personalized Hydration for Peak Performance
Hydration stands as a cornerstone of health, especially amidst the sweltering heat of summer. But did you realize that your genetic composition could influence your hydration requirements?
Discovering the nuances within your genetic makeup unveils insights into how your body manages fluid balance and electrolyte regulation. By harnessing this knowledge, you can craft a bespoke hydration regimen, ensuring you stay refreshed and invigorated throughout your summer escapades.
2. Genetic Insights for Skin Protection
Shielding your skin from the sun's potent rays is paramount for a carefree summer. While sunscreen is a go-to defense, did you know that your genetic blueprint plays a pivotal role in your skin's response to UV exposure?
Exploring genetic variations linked to skin pigmentation and DNA repair mechanisms empowers you to tailor your sun protection ritual. From selecting the ideal SPF potency to choosing sunscreen formulas fortified with antioxidants, personalized sun care ensures your skin remains radiant and resilient throughout the sunny season.
3. Personalized Strategies to Combat Heat-Related Challenges
Heat-induced ailments such as exhaustion and heatstroke pose significant threats during summer's scorching embrace. Yet, beyond keeping cool and hydrated, delving into your genetic predispositions uncovers crucial insights into your susceptibility to heat-related complications.
By understanding how genetic variances influence your body's heat regulation mechanisms, you can enact targeted preventive measures. Whether it's adjusting outdoor activities to avoid peak heat hours or seeking refuge in cool sanctuaries, personalized strategies shield you from summer's unforgiving heat.
4. Genetic Guidance for Allergy and Asthma Management
Summer's bloom often heralds the onset of allergies and asthma exacerbations, casting shadows over outdoor pursuits. However, by embracing your genetic heritage, you gain invaluable tools to manage these conditions effectively.
Exploring genetic markers associated with allergic responses and respiratory ailments informs tailored treatment approaches. From precision medications to avoidance strategies, personalized interventions empower you to breathe easy and revel in the joys of summer without restraint.
Empower Your Summer Odyssey with Genetic Wisdom
As you embark on your summer sojourn, remember that knowledge is the cornerstone of empowerment. By unraveling the mysteries encoded within your genetic fabric, you seize control of your well-being, crafting a summer suffused with vitality, resilience, and unbridled joy.
So, embrace the wisdom nestled within your genetic blueprint, bask in the cool embrace of summer, and sculpt this season into your healthiest and most exhilarating chapter yet! ☀️💪
References :
https://www.linkedin.com/pulse/shedding-light-age-related-macular-degeneration-february-kjqjc
February marks Age-Related Macular Degeneration Awareness Month, a crucial time to shed light on this condition impacting millions worldwide. With prevalence rates ranging from 1.2% to 4.7% in India alone among individuals over 40, understanding AMD becomes paramount.
What Exactly is AMD?
Age-related macular Degeneration (AMD) is a condition characterized by the deterioration of the central portion of the retina, primarily due to aging. This leads to vision loss in the central visual field, affecting activities like reading, cooking, and driving while preserving peripheral vision.
Recognizing the Signs
Symptoms of AMD may not be immediately noticeable, often progressing gradually or affecting both eyes. Challenges with central vision tasks, distorted or missing lines, dimmed vision in low light, and difficulty recognizing faces are common indicators.
Key Facts for Prevention
Taking proactive steps to prevent AMD is crucial. Here are some key insights:
- Shield Your Eyes: UV-A radiation from sunlight contributes to oxidative damage in the retina, leading to AMD. Consistently wearing sunglasses when outdoors helps mitigate this risk.
- Embrace a Healthy Diet: Obesity and high cholesterol are linked to AMD progression. Adopting a Mediterranean diet rich in antioxidants from fruits, vegetables, and legumes can slow down its advancement.
- Gender Disparities: While AMD isn't gender-exclusive, studies show a higher prevalence among women. Understanding this discrepancy aids in tailored prevention strategies.
- Kick the Habit: Smokers face significantly higher risks of developing AMD. Quitting smoking drastically reduces this risk.
- Stay Active: Regular physical activity correlates with decreased chances of AMD onset and progression. Incorporating exercise into your routine is beneficial for eye health.
- Regular Eye Checkups: Early detection is key. Routine visits to your Ophthalmologist, in-person or virtually, facilitate early screening and better long-term management.
Exploring Genetic Insights
Genetic testing, like MyDNA Gene Proactive, offers a proactive approach to understanding individual susceptibility to AMD. Post-test consultations with genetic counselors ensure comprehensive comprehension of results and potential risks.
While there's no cure for AMD, early detection, lifestyle modifications, and personalized care can effectively manage symptoms and slow progression.
For those seeking proactive measures against AMD, MyDNA Gene Proactive provides invaluable genetic insights. To learn more about this groundbreaking test, visit our website at mydna.lordsmicrobiotech.com
By raising awareness and embracing preventive measures, we can collectively combat AMD and safeguard our vision for years to come.
This blog is brought to you by MyDNA, your trusted partner in preventive healthcare and genetic wellness.
References :
https://www.linkedin.com/pulse/shedding-light-age-related-macular-degeneration-february-kjqjc
In the vast tapestry of India's health landscape, a stark and alarming reality is unfolding – one in nine individuals is now likely to face the formidable battle against cancer in their lifetime. The National Cancer Registry Programme, India, has projected a disconcerting 12.8% increase in cancer incidence by 2025 compared to 2020. This surge demands our immediate attention and a proactive approach towards understanding and mitigating the risk factors associated with this insidious disease.
The Silent Threat:
Cancer, often referred to as the silent killer, operates stealthily, making its presence felt only when it's too late. The increasing incidence of cancer cases in India paints a grim picture, signaling a health crisis that cannot be ignored. It is imperative that we confront this menace head-on, armed with knowledge and preemptive measures.
Genetic Testing: A Powerful Weapon in the Fight Against Cancer
Amidst the rising tide of cancer cases, there exists a powerful tool that can provide individuals with a crucial advantage in their battle against this formidable adversary – genetic testing. Genetic testing empowers individuals to identify their genetic predisposition to certain types of cancer, allowing for proactive measures to be taken in managing their health.
Understanding the Role of Genetic Testing:
Genetic testing involves the analysis of an individual's DNA to identify specific changes or mutations that may indicate an increased risk of developing cancer. Armed with this knowledge, individuals can make informed decisions about their lifestyle, screening routines, and treatment options.
The Urgency of Genetic Testing:
The statistics speak for themselves – the risk of developing cancer is on the rise, and we cannot afford to be complacent. Genetic testing offers a unique opportunity to understand one's inherent susceptibility to cancer and take preemptive action. By identifying genetic risks early on, individuals can tailor their health management strategies, adopt lifestyle changes, and undergo more frequent screenings, significantly improving their chances of early detection and successful treatment.
Persuasion to Act:
In the face of this escalating health crisis, the question is not whether one can afford to undergo genetic testing, but rather, can one afford not to? The preemptive power of genetic testing is a lifeline that can save lives, offering a shield against the ever-looming threat of cancer.
It is time for each one of us to take control of our health destinies. By embracing genetic testing, we arm ourselves with knowledge, the most potent weapon against the stealthy onset of cancer. Let us not be mere spectators in this battle but proactive participants, taking charge of our well-being and ensuring a healthier future for ourselves and generations to come.
Conclusion:
The surge in cancer cases in India is a clarion call for action. Genetic testing is not just a diagnostic tool; it is a beacon of hope, offering the promise of early detection and effective prevention. In the face of this alarming rise in cancer incidence, let us not be passive victims but empowered individuals who choose knowledge, precaution, and a healthier tomorrow. The time to act is now mydna.lordsmicrobiotech.com – undergo genetic testing and stand resilient against the silent threat that cancer poses to our lives.
References :
https://www.linkedin.com/pulse/unveiling-silent-threat-alarming-rise-cancer-cases-india-yavec

As we step into the month of January, we collectively observe Thyroid Awareness Month – a dedicated time to shed light on the pivotal role played by the thyroid in our overall health and to raise awareness about the various disorders that can impact it. In India alone, studies estimate that approximately 42 million people are diagnosed with thyroid diseases, underscoring the significance of understanding and prioritizing thyroid health.
Unraveling the Thyroid's Role
The thyroid, resembling a small butterfly-shaped gland in the neck, orchestrates critical bodily functions such as metabolism, energy levels, and hormone balance. Conditions like hypothyroidism and hyperthyroidism, characterized by imbalances in hormone production, are particularly prevalent, affecting millions.
Hypothyroidism manifests as inadequate hormone production, resulting in symptoms like fatigue, weight gain, and sensitivity to cold temperatures. On the other hand, hyperthyroidism involves the excessive production of thyroid hormones, leading to an overactive metabolism and symptoms such as weight loss, increased heart rate, irritability, and heat intolerance.
Thyroid Awareness Month: A Call to Action
This month serves as a reminder to prioritize thyroid health. Here are actionable steps you can take:
- Recognize Symptoms: Familiarize yourself with signs such as unexplained weight changes, fatigue, sensitivity to cold, muscle weakness, and irregular bowel movements.
- Balanced Diet: Incorporate iodine-rich foods like seafood, dairy products, and iodized salt, along with selenium-rich foods such as Brazil nuts and sunflower seeds.
- Physical Activity: Regular exercise supports metabolic health and may positively impact thyroid function. Stress reduction techniques like meditation and yoga can also contribute.
- Regular Check-ups: Schedule routine health check-ups and opt for a Thyroid Profile blood test, including T3, T4, and TSH levels, to assess thyroid function.
Empowering with Genetic Insights
Early diagnosis remains pivotal, and the MyDNA Geneproactive test offers genetic analysis of Single Nucleotide Polymorphisms (SNPs) related to hypothyroidism. SNPs are tiny changes in the DNA code, making each person unique. Armed with this knowledge, individuals can collaborate with genetic counselors to implement personalized lifestyle changes and proactive healthcare measures.
By embracing personalized adjustments, individuals can potentially mitigate the impact of genetic predispositions, optimizing overall thyroid health. Unveiling this genetic information empowers individuals to gain a deeper understanding of their risk factors and take proactive steps toward preventive healthcare.
In navigating the intricate landscape of thyroid health, awareness and personalized care emerge as our compass. Let this Thyroid Awareness Month be a catalyst for informed choices, fostering a journey towards holistic well-being and a brighter, healthier future.
References :

In the ever-evolving landscape of health and wellness, dietary advice seems to be everywhere. From trendy diets to well-meaning recommendations, the idea of a one-size-fits-all diet has permeated popular culture. However, the more we delve into the intricate world of genetics, the clearer it becomes that our individual genetic makeup plays a significant role in how our bodies respond to different foods. In this blog post, we will explore the concept of unique genetics and its impact on diet, debunking the myth that one universal diet can cater to the diverse needs of every individual.
Understanding Genetic Diversity:
The human genome is a marvel of complexity, comprising a vast array of genes that influence our traits, behaviors, and, importantly, our metabolism. Genetic variations are inherent, contributing to the diversity observed in how individuals process and utilize nutrients. For example, some people may metabolize carbohydrates more efficiently, while others thrive on a higher fat intake. Recognizing and embracing this diversity is key to tailoring diets that truly work for each person.
The Fallacy of One-Size-Fits-All Diets:
Despite the popularity of diets proclaiming universal benefits, such as ketogenic, paleolithic, or vegetarian, it's crucial to acknowledge that what works wonders for one person may not yield the same results for another. Genetic factors such as metabolism, insulin sensitivity, and nutrient absorption can vary significantly among individuals. This means that adhering strictly to a particular dietary regimen without considering individual genetic nuances may lead to suboptimal results or even health issues.
The Role of Nutrigenomics:
Nutrigenomics, the study of how individual genetic variations influence responses to nutrients, has shed light on the intricate relationship between genetics and diet. Through advancements in genetic testing, individuals can gain insights into their unique genetic makeup and how it may impact their nutritional needs. Understanding one's genetic predispositions allows for a more personalized approach to diet and lifestyle choices.
Tailoring Diets to Genetic Profiles:
Rather than subscribing to generic diet plans, an emerging trend in the health and wellness industry involves tailoring nutrition advice to individual genetic profiles. For instance, a person with a genetic predisposition for lactose intolerance might find a lactose-free diet more suitable, while someone with a high metabolic rate may benefit from a balanced macronutrient intake. Personalized nutrition takes into account factors such as food sensitivities, nutrient requirements, and metabolic tendencies.
In the pursuit of optimal health and wellness, recognizing and celebrating the uniqueness of our genetics is paramount. The myth of the one-size-fits-all diet is gradually giving way to a more personalized and nuanced approach to nutrition. Embracing the knowledge gleaned from nutrigenomics allows individuals to make informed dietary choices that align with their genetic predispositions, promoting not only better health but also a deeper understanding of the intricate interplay between genes and nutrition. As we navigate the ever-expanding realm of personalized health, the message is clear: our genes shape our dietary needs, and acknowledging this diversity is the key to unlocking our individual paths to well-being.
References :
https://www.linkedin.com/pulse/unraveling-myth-unique-genetics-fallacy-one-size-fits-all-z28dc%3FtrackingId=a67rLHn1QRLSVJVhUaDDFw%253D%253D/?trackingId=a67rLHn1QRLSVJVhUaDDFw%3D%3D

As winter blankets the world in a serene layer of frost, our focus naturally shifts towards staying warm and healthy. While the season encourages cozy blankets and warm beverages, did you know that your genetic makeup may also play a role in how your body responds to the winter chill? In this blog, we'll explore the intriguing connection between winter health and genetics.
References :
https://www.linkedin.com/pulse/embracing-winter-wellness-unveiling-genetic-connection-rbhtc%3FtrackingId=LN%252BXz14HQ6eGz9r3p9rctA%253D%253D/?trackingId=LN%2BXz14HQ6eGz9r3p9rctA%3D%3D

People genetically predisposed to have high LDL cholesterol are at an increased risk for coronary heart disease even if their cholesterol levels are modestly elevated, as per the recent study published in Circulation.
Familial hypercholesterolemia, a genetic disorder causes elevated LDL (sometimes called "bad") cholesterol from birth and often leads to coronary heart disease at a young age.
Familial hypercholesterolemia affects around 1 in 250 people in the U.S., as per the Centers for Disease Control and Prevention.
In the study, the study group analyzed data from more than 21,000 participants with no existing coronary heart disease who underwent whole genome sequencing as part of six previously conducted population-based cardiovascular studies. Investigators noted any variations in genes typically associated with familial hypercholesterolemia, as well as each person's LDL cholesterol levels and other risk factors, including smoking status and BMI.
After following up with the participants for about 20 years later, the study group found that people with high LDL cholesterol and the associated familial hypercholesterolemia genetic variants were nearly four times more likely to have coronary heart disease than those without the variants, according to the study.
Additionally, the presence of familial hypercholesterolemia genetic variations was associated with a higher risk of coronary heart disease, even when LDL cholesterol levels were only modestly elevated.
Pooling together genetic and cholesterol data, and then outcomes data from these six population-based studies, high cholesterol is worse than low cholesterol, but high cholesterol that's related to one of these single-gene disorders is particularly risky and even riskier than the polygenic variety.
"These findings will beg the question, 'Does this mean we need to do genetic testing in everybody who has high cholesterol levels?' I think it suggests that we might want to do more of it, and particularly in familial settings," the author said.
"If we identify one person, we want to do what we call cascade screening: Find other people in the family who might be affected by this so that we understand all of the risk in the family. But in general, the tools and the steps that we'll take to control that cholesterol will be the same regardless of whether I know they have an underlying single gene defect or not."
References :
- Adapted from : Yiyi Zhang et al, Association of Severe Hypercholesterolemia and Familial Hypercholesterolemia Genotype With Risk of Coronary Heart Disease, Circulation (2023). DOI: 10.1161/CIRCULATIONAHA.123.064168 Accessed on 25th June ‘2023

A study group from Melbourne, Australia conducted a Prospective study of women with invasive or high grade in situ breast cancer and unknown germline status and the same discussed at the multidisciplinary team meeting. Women were recruited to the pilot (12 June 2020 - 22 March 2021) and expansion phases (17 October 2021 - 8 November 2022) of the Mutational Assessment of newly diagnosed breast cancer using Germline and tumour genomICs (MAGIC) study to determine the feasibility of universal genetic testing of women with newly diagnosed breast cancer, to estimate the incidence of pathogenic gene variants and their impact on patient management, and to evaluate patient and clinician acceptance of universal testing.The study group identified pathogenic germline variants in 31 of 474 expanded study phase participants (6.5%), including 28 of 429 women with invasive breast cancer (6.5%).
18 of the 31 did not meet current genetic testing eligibility guidelines (probability of a germline pathogenic variant ≥ 10%, based on CanRisk, or Manchester score ≥ 15). In 24 of 31 women clinical management was changed for after identification of a pathogenic variant. Further, 68 women who underwent genetic testing outside the study, 44 of 542 women carried pathogenic variants (8.1%).
“Acceptance of universal testing was high among both patients (90 of 103, 87%) and clinicians; no decision regret or adverse impact on psychological distress or cancer-specific worry were reported as per the investigators”
Finally, universal genetic testing following the diagnosis of breast cancer detects clinically significant germline pathogenic variants that might otherwise be missed because of testing guidelines. Routine testing and reporting of pathogenic variants is feasible and acceptable for both patients and clinicians.
References :
- Adapted from : De Silva DL, Stafford L, Skandarajah AR, Sinclair M, Devereux L, Hogg K, Kentwell M, Park A, Lal L, Zethoven M, Jayawardana MW, Chan F, Butow PN, James PA, Mann GB, Campbell IG, Lindeman GJ. Universal genetic testing for women with newly diagnosed breast cancer in the context of multidisciplinary team care. Med J Aust. 2023 May 1;218(8):368-373. doi: 10.5694/mja2.51906. Epub 2023 Apr 2.